PedMedDev - The Hub for Pediatric Medical Devices
The overall goal of the PedMedDev Hub is to promote and improve medical devices for our children.
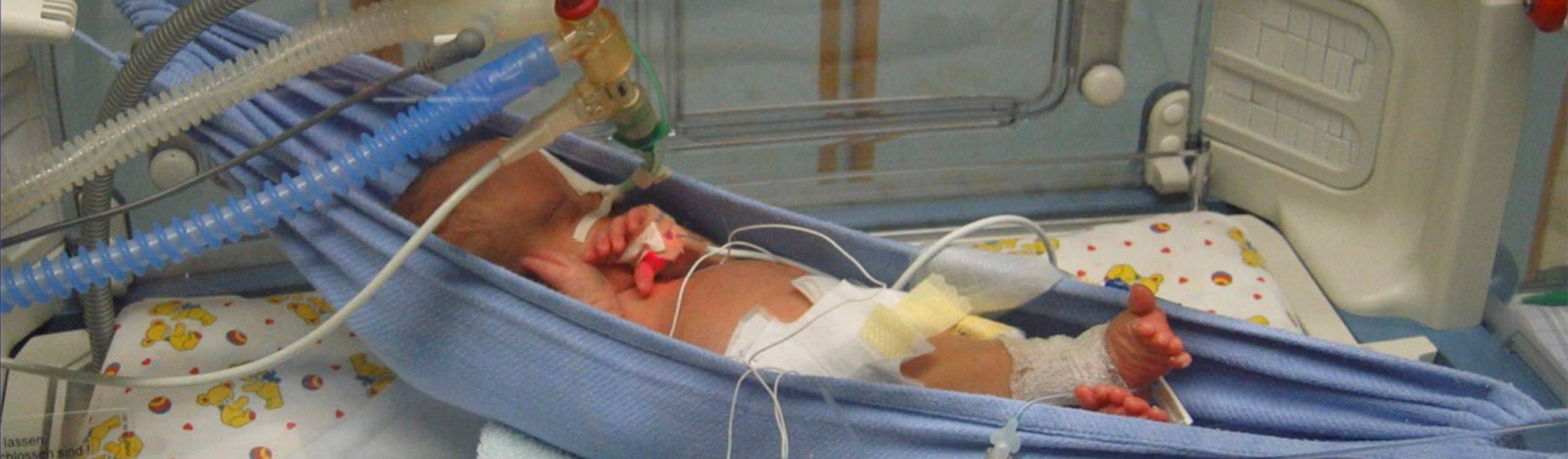
Regulatory requirements, high development costs, a small market share and insufficient remuneration hinder the development of child-centered medical technology, although it is precisely they, and with them society, that would benefit most.
The PedMedDev Hub is the hub for linking people, resources and infrastructures. By combining synergistically the potential of all stakeholders like patients, clinicians, scientists, engineers and medical device manufacturers , the above-mentioned hurdles in the development of medical devices for children can be overcome. This includes the identification and evaluation of clinical needs and the design and development of creative solutions and processes as well as the transfer to the clinic and thus to the patient - both with national and European reach.
The PedMedDev Hub will intensify the existing activities and realize the network of competencies to enable strategic initiatives to accelerate safe, efficient and cost-effective innovation.
In particular, the current new technologies (e. g. 3D printing, rapid prototyping, digitalization in healthcare, AI, sensor technology, nano devices) are creating major opportunities for paediatric medical devices.
The PedMedDev - Project is supported by the Damp Stiftung.
Testimonials
Prof. Dr. Chris Paton
University of Oxford, UK
Dr. Gerhard Pohlmann
Fraunhofer-Institut für Toxikologie und Experimentelle Medizin ITEM, Hannover
Dr. Heike Wachenhausen
Wachenhausen Law, Lübeck, Germany
Prof. Dr. Lutz Wünsch
Klinik für Kinderchirurgie am UKSH Lübeck, Germany
Prof. Dr. Peter Ewert
Deutsches Herzzentrum Munich, Germany
Prof. Dr Gwenyth Fischer
University of Minnesota, Masonic Children's Hospital, Minneapolis, USA
Prof. Dr. Egbert Herting
Klinik für Kinder- und Jugendmedizin UKSH Lübeck, Germany
Prof. Dr. Anne-Francoise Spinoit
University of Gent, Belgium
Prof. Dr. Mark Turner
University of Liverpool, UK
Prof. Dr. Annelie Weinberg
Medizinische Universität Graz, Austria